
Agriculture
November 9, 2023

Updated on November 9, 2023
·Created on August 6, 2016
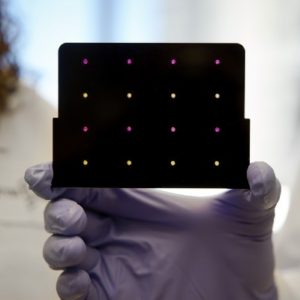
The Rapid Assessment of Infectious Diseases (RAID) Device is a portable, reusable device that exploits the magnetic properties of disease biomarkers to detect diseases in less than 1 minute.
The Rapid Assessment of Infectious Diseases (RAID) Device is a prototype portable, reusable device that exploits the magnetic properties of biomarkers to detect infectious diseases in less than 1 minute. It can detect fever-like diseases such as dengue and upper respiratory infections such as pneumonia.
Market Suggested Retail Price
$1,000.00
Target Users (Target Impact Group)
Distributors / Implementing Organizations
The device is distributed by regional partners including Bosch Healthcare and Denka.
Manufacturing/Building Method
The product is manufactured in Boston, USA. The electrical circuit boards and precision machining of the optical mount are out sourced while all assembly is done in house. Interview with designer, September 2020
Intellectural Property Type
Trade Secret
User Provision Model
The device can be purchased from a partner (Bosch Healthcare or Denka), from a clinical partner under one of these two main partners, or through the product website. Interview with designer, September 2020
Distributions to Date Status
10 first generation devices and 8 second generation devices have been distributed. Interview with designer, September 2020
Design Specifications
Awaiting response from the manufacturer.
Technical Support
Contact the manufacturer.
Replacement Components
Device consumables include rechargeable batteries and cuvettes for the blood samples. The manufacturer supplies replacement components and provides maintenance services. Interview with designer, September 2020
Lifecycle
5+ years Interview with designer, September 2020
Manufacturer Specified Performance Parameters
Designer-specified performance targets include: affordable, quick, and simple.
Vetted Performance Status
The manufacturer ran a clinical trial in India in 2013 on 250 patients. No third party testing has been completed.
Safety
Care must be taken when taking the blood samples.
Complementary Technical Systems
Device requires batteries to function.
Academic Research and References
Awaiting response from the manufacturer.
Compliance with regulations
The device complies with individual partner regulations. Interview with designer, September 2020
Other Information
Winner of MIT 100K, MassChallenge Boston, Cupid's Cup, and 43 North.

Agriculture
November 9, 2023
Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Agriculture
November 9, 2023
Agriculture
November 9, 2023

Agriculture
November 9, 2023
Agriculture
November 9, 2023
Have thoughts on how we can improve?
Give Us Feedback
The DDG malaria device offers the promise of extremely rapid malaria detection based on plasmodium hemoglobin digestion into hemozoin precipitates that can be detected with an optical reader. Other manufacturers have shown that the use of a portable powered device in a remote setting is a successful approach (Hemocue).
The technology while compelling needs field trials to assess performance relative to blood smear and blood RDTs. There is also some concern in the literature about the limitations of hemozoin as an indicator (see references below). Although it is early days for the device manufacturer, there should be optimism that these potential limitations will be overcome with continued development and field testing.
Rebelo, Maria, Howard M. Shapiro, Teresa Amaral, José Melo-Cristino, and Thomas Hänscheid. 2012/7. “Haemozoin Detection in Infected Erythrocytes for Plasmodium Falciparum Malaria diagnosis—Prospects and Limitations.†Acta Tropica 123 (1): 58–61.
Delahunt, Charles, Matthew P. Horning, Benjamin K. Wilson, Joshua L. Proctor, and Michael C. Hegg. 2014. “Limitations of Haemozoin-Based Diagnosis of Plasmodium Falciparum Using Dark-Field Microscopy.†Malaria Journal 13 (April): 147.