
Agriculture
November 9, 2023

Updated on November 9, 2023
·Created on September 4, 2019
CURE Prosthetics is an EMG controlled prosthetic arm.
CURE Prosthetics is a prosthetic arm that is EMG (Electromyography) sensor controlled. The arm is built through 3D printing by CURE, a startup in Tunisia looking to developed personalized bionic hands for an affordable price. This is combined with virtual reality for physical rehabilitation.
Market Suggested Retail Price
$3,000.00
Target Users (Target Impact Group)
Distributors / Implementing Organizations
Manufacturing/Building Method
The prothesis is 3D printed and sensors are added. The arm can be customized to the user. It is manufactured in Tunisia where CURE is based.
Intellectural Property Type
Patent Protected
User Provision Model
The prosthesis can be purchased from CURE, which is looking to develop a business to business model with national and international distributors, government agencies, and hospitals.
Distributions to Date Status
Three, having just completed testing phase in July 2019 Interview with representative
Design Specifications
The prosthesis is designed to be affordable and to be manufactured in Tunisia. It is designed for below the elbow/transradial amputees. The arm uses myoelectric sensors and is made of a corn-based plastic.
Technical Support
CURE provides one month of AR and VR based training for users.
Replacement Components
Replacement components include components such as fingers if needed. For users, replacement components are two batteries.Interview with representative
Lifecycle
At least three years, currently working to expand to five years Interview with representative
Manufacturer Specified Performance Parameters
Vetted Performance Status
Completed internal testing July 2019 Interview with representative
Safety
Temperature and water considerations: Cannot be wet, cannot be near fire. No skin allergy concerns Interview with representative
Complementary Technical Systems
CURE provides AR and VR based training for users.
Academic Research and References
None
Compliance with regulations
None
Other Information
None

Agriculture
November 9, 2023
Agriculture
November 9, 2023
Implemented by
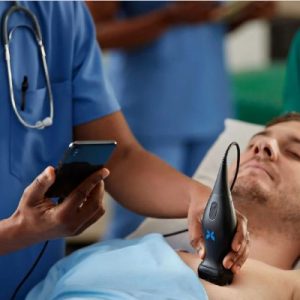
Butterfly iQ

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Implemented by
Ecofiltro

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Have thoughts on how we can improve?
Give Us Feedback