
Agriculture
November 9, 2023
Updated on November 9, 2023
·Created on August 21, 2020
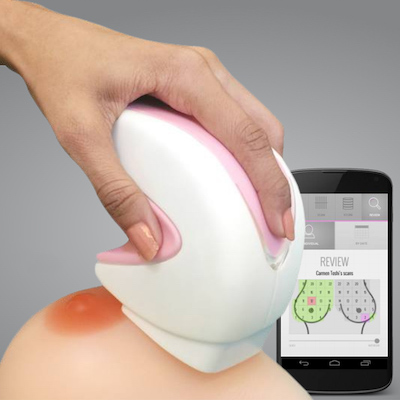
iBreastExam is a rapid diagnostic for early-stage detection of breast abnormalities.
iBreastExam is a low cost, battery-powered, handheld rapid diagnostic device used for the identification of breast abnormalities and early detection of breast cancer. The device is radiation-free and is non-invasive. The product is being commercially distributed in over 25 countries across Africa and South Asia.
Market Suggested Retail Price
$9,981.00
Target Users (Target Impact Group)
Distributors / Implementing Organizations
GE Healthcare is the primary distributor. iBreastExam has partnered with 40+ private clinics, governmental institutions, non-profits, and corporate social responsibility initiatives to enable the use of the product and breast examinations.
Manufacturing/Building Method
The product is mass-manufactured in India.
Intellectural Property Type
Patent Protected
User Provision Model
As of now, the product has been used through large-scale governmental initiatives as partnerships between the manufacturer and governmental, national, or international institutions. Users can request a demo through the manufacturer's website.
Distributions to Date Status
As of July 2020, 200+ products have been installed in health care settings and 300,000+ women have been screened.
Design Specifications
The iBreastExam is a handheld device for breast abnormality detection. The device uses a ceramic sensor technology that is able to detect subtle variations in tissue elasticity, which corresponds to the detection of lumps in the breast tissue. The device is battery operated, wireless, and radiation-free. A typical scan takes about 5 minutes and the data from the device is immediately sent to a smartphone, where user data is stored. The device goes over the four quadrants of the breast during an exam and colours are used to communicate possible abnormalities to the operator. Green represents normal tissue and red indicates a possible abnormality and need for further assessment. The colours are shown both by an LED on the device itself as well as on a breast map on the smartphone. The device can detect tissue abnormalities as small as 3-5 mm. It takes 4-8 hours to train a health care worker on the operation of the device, after which the worker takes a test before being certified.
Technical Support
Provided by the manufacturer. Regional distributors also provide product servicing.
Replacement Components
There are no replaceable components.
Lifecycle
2-5 years
Manufacturer Specified Performance Parameters
Manufacturer specified performance targets include portability, low cost, scalable, standardized, accessible, and easy to use.
Vetted Performance Status
There have been three clinical studies using the iBreastExam which showed the following approximate averages: 86% sensitivity, 90% specificity, 63% PPV, 89% NPV.
Safety
Device safety training is included in the general device training provided to health care workers.
Complementary Technical Systems
A smartphone is required for data transmission and saving. A reliable power source is required to recharge the battery in the device.
Academic Research and References
S. P. Somashekhar, R. Vijay, R. Ananthasivan, and G. Prasanna, Noninvasive and Low-Cost Technique for Early Detection of Clinically Relevant Breast Lesions Using a Handheld Point-of-Care Medical Device (iBreastExam): Prospective Three-Arm Triple-Blinded Comparative Study, Indian J. Gynecol. Oncol., vol. 14, no. 2, pp. 1–6, Jun. 2016, doi: 10.1007/s40944-016-0057-1.
R. B. Broach, R. Geha, B. S. Englander, L. DeLaCruz, H. Thrash, and A. D. Brooks, A cost-effective handheld breast scanner for use in low-resource environments: A validation study, World J. Surg. Oncol., vol. 14, no. 1, p. 277, Oct. 2016, doi: 10.1186/s12957-016-1022-2.
Compliance with regulations
The iBreastExam has CE and Unicert ISO13485 certification and has been cleared by the FDA.
Other Information
The iBreastExam has won several awards: 2019 Lyfebulb-Helsinn Oncology Innovation Award, 2019 Best Pitch Innov8 Talks Arab Health Award, 2018 Bayer Foundation Grants 4 Impact Award.

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Agriculture
November 9, 2023

Agriculture
November 9, 2023
Implemented by
NRSRelief

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Have thoughts on how we can improve?
Give Us Feedback