Agriculture
November 9, 2023
Updated on November 9, 2023
·Created on August 27, 2015
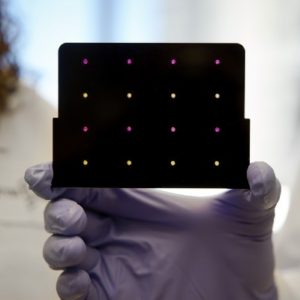
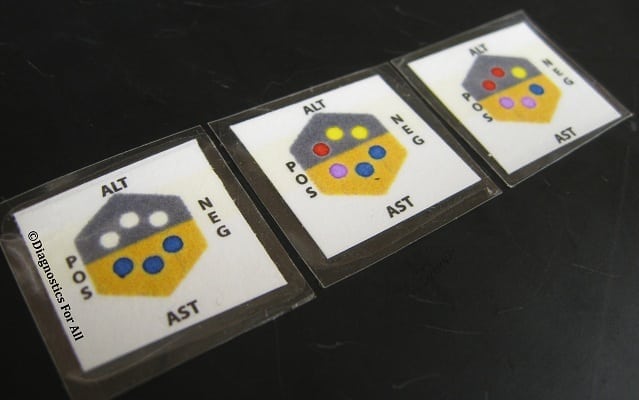
Diagnostics for All (DFA) Paper-Based Liver Test is a prototype low-cost, paper-based diagnostic testing for liver enzymes.
Diagnostics for All (DFA) Paper-Based Liver Test is a prototype low-cost, paper-based diagnostic testing for liver enzymes. A fingerprick of blood and 15 minutes will give healthcare providers an assessment of the patient’s liver health.
Target Users (Target Impact Group)
Distributors / Implementing Organizations
Distributed by manufacturer
Intellectural Property Type
Patent Protected
Design Specifications
Chemically versatile : Patterned paper technology can be applied to generate immunoassay, electrochemical analysis, clinical chemistry analysis and molecular diagnosis. Easy-to-use and easy-to-read : Finger prick samples are sufficient to produce results that do not require a syringe, clean water, or sample preparation. Results are quickly displayed for immediate clinical decision making. Disposable : DFA's pattern-based paper-based devices are disposable, and can be easily and safely disposed of through incineration, ensuring that medical waste issues around the world do not grow.
Technical Support
Provided by the manufacturer
Replacement Components
N/A
Lifecycle
Single-use product
Manufacturer Specified Performance Parameters
Designer specified performance targets include: portable, and low-cost.
Vetted Performance Status
Waiting for response from manufacturer
Safety
No known safety hazards are related to this product.
Complementary Technical Systems
None
Academic Research and References
Andres, W., Scott, T., et al., 2010, Programmable diagnostic devices made from paper and tape. Royal Society of Chemistry, 10, pp. 2499 – 2504.
Mohidus, K., George, T., et al., 2010, Paper Diagnostic for Instantaneous Blood Typing. Analytical Chemistry, 82 (10),pp. 4158-4164.
Compliance with regulations
DFA has an exclusive worldwide license from Harvard for medical and other technical applications.
Other Information
None

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Implemented by
Aquagenx

Agriculture
November 9, 2023
Implemented by
mWater
Agriculture
November 9, 2023

Agriculture
November 9, 2023
Implemented by
Nanyang Technological University (NTU) Singapore

Agriculture
November 9, 2023
Implemented by
HACH

Agriculture
November 9, 2023
Have thoughts on how we can improve?
Give Us Feedback
Buy-one-give-one is charity based and not a sustainable or scalable business model.
Similar to BeGirl Flexi Pads, using a reusable “sleeve” with disposable absorbent material is an interesting approach. If used properly, this could reduce the risk of infection from contaminated absorbent material. But herein also lays a risk: Not only might it be difficult for women find suited “clean” absorbent materials, sanitizing the panties properly between uses could also pose a challenge (e.g. lack of clean water, disinfectant soap etc.). User compliance to cleaning instructions could also be an issue. Improper cleaning of the panties between uses or the use of unsuited absorbents could result in the pads carrying pathogens which could have detrimental health effects, effectively posing a similar risk as traditional reusable cloth napkins made from old saris etc. I’d be reluctant to trade off health benefits in menstrual health management.
Again, make sure the links target a new window or tab.
Both coverage to obtain orders and logistics to ensure delivery must be designed to function effectively in the specified environment. The stated distribution of “Africa†and “India†is unclear as to whether these products will be used in capital cities or more broadly in regional centers or even up country clinics. The product information simply says that distribution will be made through “Direct Sales†implies that MSF will be staffing a sales team. It is unclear if such a strategy is practical. Given the size and weight of this type of product and the utility connections to water supplies etc. trained installation specialists and technicians are required to ensure proper installation. More detail is required to define and effective strategy and ability to scale.
Stating following a design toolkit shouldn’t be in ‘Compliance with regulations’. Consider removing ?