
Agriculture
November 9, 2023
Updated on November 9, 2023
·Created on July 27, 2017
Aina is a diagnostic device on smartphones that can analyzes HbA1c, blood glucose, hemoglobin, creatinine, and lipids from a capillary blood sample.
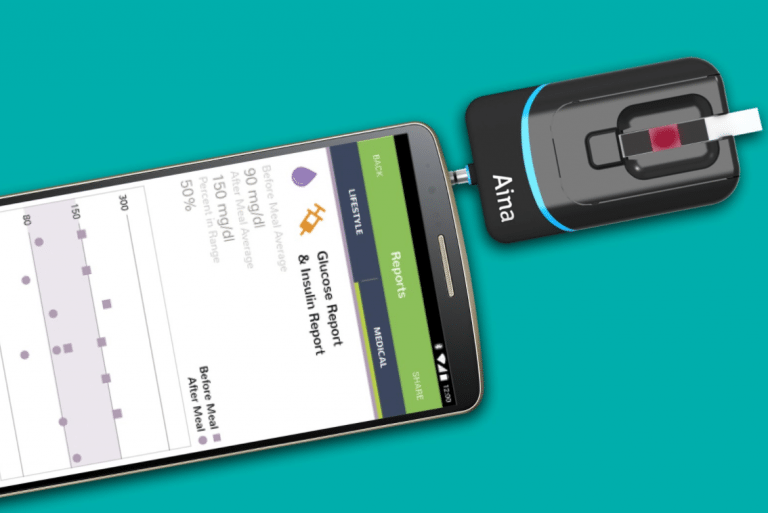
Aina is a diagnostic sensor and smartphone application that plugs into any smartphone and analyzes HbA1c, blood glucose, hemoglobin, creatinine, and lipids from a capillary blood sample in only minutes. The device can be used by patients for self-monitoring as well as by healthcare providers for mass screening and point-of-care testing.
Aina has also developed the Habits Program, based on the landmark Diabetes Prevention Program, to deliver digital coaching and support to patients recently diagnosed with pre-diabetes or type II diabetes.
Target Users (Target Impact Group)
Distributors / Implementing Organizations
The manufacturer distributes his own product, Hospitals like Narayana Health Hospital in Bangalore, India are implementing the product
Manufacturing/Building Method
The product is also Mass Produced. The company is headquartered in Boston with an ISO13485 certified manufacturing site in India
Intellectural Property Type
Patent Protected
User Provision Model
Patients can procure the Jana Care Aina from health clinics and hospitals or purchase it directly from the manufacturer and download the companion app from the Google Play or the Apple Store. The healthcare workers also use the device to directly test patients. Customers can also request a demo from the manufacturers website
Distributions to Date Status
50,000 patients in India as of February 2017.
Design Specifications
The Jana Care Aina Device is a blood glucometer that uses existing colorimetric test strips and plugs into any smartphone via the headphone jack. Blood readings are communicated to an accompanying mobile app, which allows pre-diabetic users to monitor consistently their glucose levels as well as weight, food intake, and physical activity. The product works with iOs and Android devices.
The Aina device HbA1c test utilizes a method called boronate affinity. The Aina HbA1c Test Kit consists of test strips, reagents, wash buffers, capillary tubes for collection of samples, and pipette tips. The reagent contains a lysing agent and a blue boronic acid conjugate. When blood is added to the reagent, the erythrocytes are lysed and all hemoglobin precipitates. The boronic acid conjugates binds to the glycosylated hemoglobin. An aliquot of the reaction mixture is applied to the test strip and all the precipitated hemoglobin, conjugate-bound and unbound, remains on top of the filter. Any unbound boronate is removed with the wash buffer. The precipitate is evaluated by measuring the blue (glycosylated hemoglobin) and the red (total hemoglobin) color intensity
respectively with the Aina Device, the ratio between them being proportional to the percentage of the glycosylated hemoglobin in the sample.
The product has the following technical specifications
Dimensions: 79.57 (L) X 36.54 (B) X 16.40 mm (H)
Weight: 0.021kg (without battery), 0.032 kg (with battery)
Power Requirements: 1.5V AAA battery
Safety: Battery powered device, tested for compliance with EN 61010-1, EN 61010-2-101 and RoHS
EMC Emissions/Immunity: Aina Device complies with applicable EMC emission requirements as per EN 61326-1:2013 and EN 61326-2-6:2013
Technical Support
Users can be supported through the habits App. The user can also visit Jana care's desk support
Replacement Components
Lifecycle
The test strips are single-use. Aida device lifecycle is unknown.
Manufacturer Specified Performance Parameters
Goals: - Early detection for diabetes, pre-diabetes and other chronic diseases - In point diagnostics and monitoring - Easy to use - Low cost
Vetted Performance Status
Method comparison to a YSI 2300 analyzer with 750 patient blood samples shows that the Jana Care Aina conforms with accuracy and repeatability requirements specified by ISO 15197:2013 standard (In vitro diagnostic test systems — Requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus). Additional test results can be found here.

Safety
The device is tested for compliance with EN 61010-1, EN 61010-2-101 and RoHS It is important to make sure hands and device are clean before use in order to prevent infection.
Complementary Technical Systems
Smartphone, testing strips, the Jana Care Habits app
Academic Research and References
Clarke, W. L., Cox, D., Gonder-Frederick, L. A., Carter, W., & Pohl, S. L. (1987). Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes care, 10(5), 622-628.
Compliance with regulations
Aina's accuracy meets ISO15197:2013 regulatory standards needed for CE and US FDA approval.
Other Information
The device also docks with the Aina Station, a point-of-care platform that can sit in a primary care office or clinic to screen patients with the goal of reduce time that patients have to wait for results from a central lab.

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Have thoughts on how we can improve?
Give Us Feedback