
Agriculture
November 9, 2023
Updated on November 9, 2023
·Created on August 31, 2021
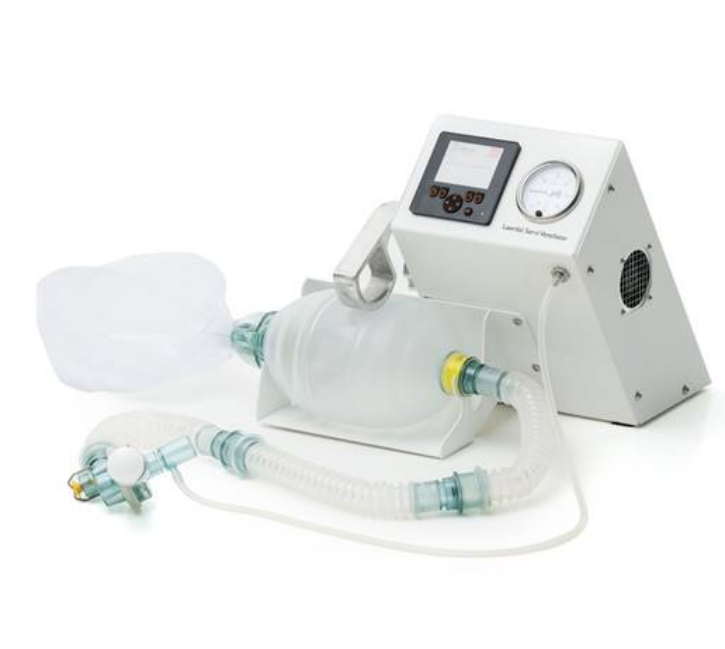
The Laerdal Servi Ventilator is an emergency invasive ventilator designed and implemented in response to the COVID-19 pandemic. Courtesy of WHO Compen
Developed in 2020 in response to the needs from the global COVID-19 pandemic, the Laerdal Servi Ventilator is an emergency invasive ventilator designed not to replace existing ventilators in over-capacity hospitals, but to offer a supplemental technology in emergency situations. As such, the design features settings for sedated patients with respiratory failure, intended to be used over a relatively short period of time on the patient.
This product was selected for inclusion in WHO’s 2021 Compendium of Innovative Health Technologies for Low‐Resource Settings.
Target Users (Target Impact Group)
Distributors / Implementing Organizations
This product was implemented by the following organizations: the Norwegian Defense Research Establishment, Edge Health Technologies AS, Laerdal Medical AS, and Servi AS.
Manufacturing/Building Method
The product is manufactured by Laerdal in Stavanger, Norway. The company produces components and and other products from their factories in Norway, Mexico, China, and USA.
Intellectural Property Type
Trademarked
User Provision Model
The emergency ventilators are purchased by European governments to then distribute to hospitals in need.
Distributions to Date Status
1000 ventilators as of 2020
Design Specifications
The Laerdal Servi Ventilator incorporates the following components: (1) oxygen tubing and reservoir kit, (2) three 28 cm extension tubes, (3) patient valve with pressure relief valve, (4) exhalation port with a diameter of 30 mm, and (5) manometer connector. There are three consumables: (6) HME filter, (7) Thomas Tube holder, and (8) adjustable PEEP valve. The control unit allows for tidal volume adjustment between 100-800 ml and ventilation rates between 5-30 per min.
Technical Support
Technical support is provided by the manufacturer.
Replacement Components
Replacement components and consumables are available for purchase from the manufacturer
Lifecycle
Unknown
Manufacturer Specified Performance Parameters
The manufacturer's goal of the rapid-development project emphasized quick production, simple to use, and simplified (but accurate) automated assisted breathing.
Vetted Performance Status
The system has been tested with test lungs to in both controlled and assisted ventilation mode, details of the performance testing and design iterations are available in this report.
Safety
The product has been designed and tested to meet patient safety standards in Norway.
Complementary Technical Systems
None
Academic Research and References
Compliance with regulations
Received the European CE (Communauté Européenne) Mark
Other Information
None

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Have thoughts on how we can improve?
Give Us Feedback