Agriculture
November 9, 2023

Updated on November 9, 2023
·Created on August 27, 2015
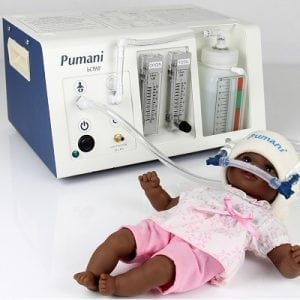
A respiratory support device for infants.
The Seattle Children’s Hospital High Oscillation Continuous Positive Airway Pressure (Sea-PAP) Device is a respiratory support technology for infants. The NeoRest Team claims that Sea-PAP improves upon conventional bCPAP by more consistently producing a range of oscillations thought to improve lung function and make it easier for an infant to breathe.
Target Users (Target Impact Group)
Distributors / Implementing Organizations
The NeoRest Team is in the process of finalizing the Sea-PAP device design, identifying manufacturers to produce the device and preparing to conduct clinical trials. It is not being distributed widely at this time.
Manufacturing/Building Method
The main Sea-PAP device body will be mass produced. The unit can be assembled on-site with a small tool kit of supplies including a continuous air supply, a breathing circuit tube, a tube for the nose and water.
Intellectural Property Type
Other
User Provision Model
Unknown
Distributions to Date Status
Prototype device which has not been distributed at scale.
Design Specifications
Technical Support
N/A
Replacement Components
Unknown
Lifecycle
The breathing and nose tubes can be used multiple times if sterilized. Disposable units can be used, however require a robust consumables supply chain.
Manufacturer Specified Performance Parameters
Unknown
Vetted Performance Status
Unknown
Safety
Unknown
Complementary Technical Systems
The Hansen Ventilator - a separate respiratory device - can be used in conjunction to treat even more premature infants with respiratory distress.
Academic Research and References
Diblasi RM, Noninvasive respiratory support of juvenile rabbits by high-amplitude bubble continuous positive airway pressure. Pediatr Res. 2010 Jun;67(6):624-9. doi: 10.1203/PDR.0b013e3181dcd580.
Diblasi RM, Nasal continuous positive airway pressure (CPAP) for the respiratory care of the newborn infant. Respir Care. 2009 Sep;54(9):1209-35.
Compliance with regulations
In October 2013, Sea-PAP received clearance from the Food and Drug Administration for use and distribution in the U.S.
Other Information
Unknown

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Agriculture
November 9, 2023

Agriculture
November 9, 2023
Agriculture
November 9, 2023

Agriculture
November 9, 2023
Implemented by
Stanford Researchers and the International Centre for Diarrhoeal Disease Research, Bangladesh
Agriculture
November 9, 2023

Agriculture
November 9, 2023
Agriculture
November 9, 2023

Agriculture
November 9, 2023
Have thoughts on how we can improve?
Give Us Feedback