
Agriculture
November 9, 2023
Updated on November 9, 2023
·Created on September 7, 2016
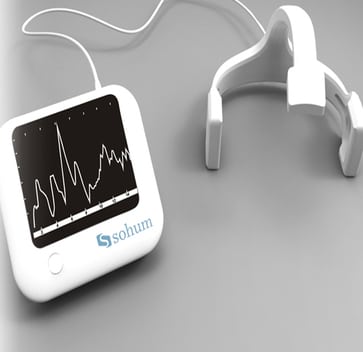
The Sohum hearing screening device is a non-invasive device that uses brainstem auditory evoked response (BAER or ABR) technology to screen neonates for hearing impairment.
The Sohum hearing screening device is a non-invasive device that uses brainstem auditory evoked response (BAER or ABR) technology to screen neonates for hearing impairment. It can screen both ears simultaneously, withstands background noise, does not require sedation, and is user-friendly for resource-constrained settings.
Target Users (Target Impact Group)
Distributors / Implementing Organizations
Manufacturing/Building Method
Sohum Innovation Labs developed the Sohum hearing device prototype, which was launched for use in 2017 by the Union Ministry of Science and Technology. They are manufactured in India, and meet ISO 13485 standards.
Intellectural Property Type
Patent Protected
User Provision Model
The device will be made available to Public Health Centers by the government. Healthcare workers will conduct screenings of neonates, and children testing positive for hearing loss will be connected to Sohum-certified audiologists for further care.
Distributions to Date Status
Unknown
Design Specifications
Sohum uses brainstem auditory evoked response (BAER or ABR) technology for testing. The device checks both ears simultaneously, is non-invasive, and doesn't require sedation. It can filter out clinic ambient noise from the test signal, and is battery operated.
Technical Support
Unknown, still at prototyping phase
Replacement Components
Batteries
Lifecycle
Unknown, still at prototyping phase
Manufacturer Specified Performance Parameters
This hearing screening device was designed to measure auditory waves via three electrodes placed on a baby's head, filter out health clinic ambient noise, be non-invasive, be user-friendly for clinics in low-resource settings, and be low-cost and portable.
Vetted Performance Status
Safety
Unknown, still at prototyping phase
Complementary Technical Systems
Battery operated
Academic Research and References
None
Compliance with regulations
The device is certified by the Ministry of Science and Technology and ISO 13485 standards.

Agriculture
November 9, 2023
Agriculture
November 9, 2023
Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023

Agriculture
November 9, 2023
Implemented by
Jorge Od├│n

Agriculture
November 9, 2023
Have thoughts on how we can improve?
Give Us Feedback